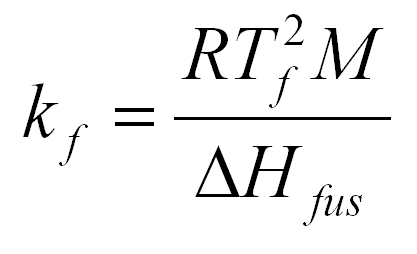
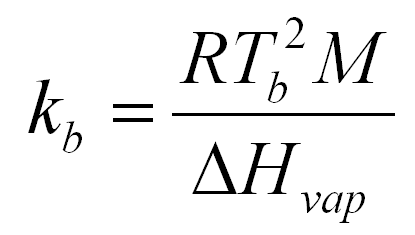
formula
1
formula
2
M is the molecular weight of the substance in question.
MHS AP Chemistry
Advanced Colligative Properties Lab
The purpose of this activity is to determine the phase change constant and enthalpy of phase change for a substance, and compare the experimental values with "literature" values. The inspirations for this lab were the CRC handbook tables of "Cryoscopic Constants" and "Ebullioscopic Constants." You may choose one of the following variations:
Variation 1: Freezing Point Depression
Variation 2: Boiling Point Elevation
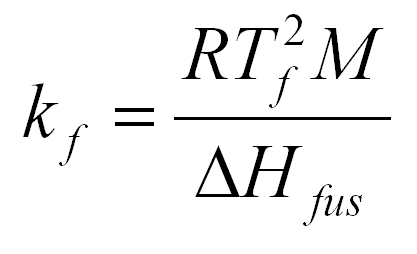 |
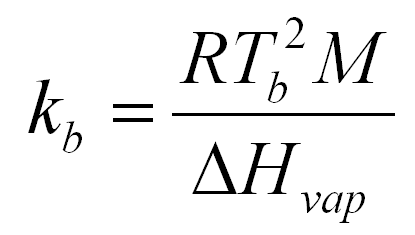 |
formula
1 |
formula
2 |
M is the molecular weight of the substance in question. |
|